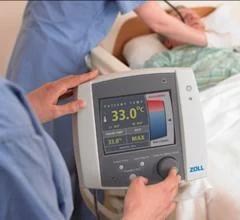
The global Therapeutic Hypothermia Systems Market size is predicted to reach USD 422.9 million by 2030 with a CAGR of 5.6% from 2025 to 2030. The therapeutic hypothermia systems market, critical for improving patient outcomes in conditions like cardiac arrest, stroke, and neonatal care, is witnessing significant developments in 2025. These systems, which lower body temperature to reduce brain injury and enhance recovery, are becoming integral to emergency and critical care settings. Recent advancements in technology, strategic partnerships, and increasing clinical acceptance are shaping the market’s trajectory. This article explores the latest trends and news driving the therapeutic hypothermia systems market, drawing on recent industry updates.
Rising Clinical Acceptance and Applications
Therapeutic hypothermia has gained traction within the medical community as a vital intervention for improving neurological outcomes. Its efficacy in post-cardiac arrest care, where controlled cooling reduces brain damage, has solidified its role in emergency medicine. Beyond cardiology, applications in neurology for stroke patients and neonatal care for hypoxic-ischemic encephalopathy are expanding. Recent studies highlight improved survival rates and reduced long-term neurological deficits, driving demand for advanced hypothermia systems in hospitals and trauma centers.
The growing recognition of hypothermia’s benefits is reflected in updated clinical guidelines. Medical societies are increasingly recommending targeted temperature management (TTM) as a standard protocol in critical care, prompting hospitals to invest in modern hypothermia systems. This shift is particularly evident in developed regions, where healthcare infrastructure supports rapid adoption of such technologies.
Technological Innovations Fueling Market Growth
Recent innovations in therapeutic hypothermia systems are enhancing their efficiency and usability. Manufacturers are developing systems that offer precise temperature control, portability, and integration with digital health platforms. For instance, cooling catheters and water blankets are gaining popularity for their ability to deliver consistent cooling with minimal invasiveness. These advancements allow clinicians to implement hypothermia protocols more effectively, even in high-pressure environments like intensive care units (ICUs).
A notable trend is the integration of artificial intelligence (AI) into hypothermia systems. AI-powered devices can monitor patient temperature in real-time, adjust cooling parameters, and predict potential complications, improving patient safety and outcomes. This technological leap is attracting investment from healthcare providers seeking to enhance their critical care capabilities. Additionally, portable cooling systems are making hypothermia therapy more accessible in smaller hospitals and during patient transport, expanding the market’s reach.
Strategic Partnerships and Market Expansion
The therapeutic hypothermia systems market is seeing increased activity through strategic partnerships and distribution agreements. In January 2024, SourceMark announced an expanded partnership with Gentherm Medical, a leading manufacturer of patient temperature management systems. This collaboration strengthens SourceMark’s role as a master supplier for Gentherm’s product lines, including Astopad (resistive warming), Norm-O-Temp (whole-body hyperthermia), and WarmAir (convective warming), which complement hypothermia systems in perioperative settings. This partnership enhances product availability in the U.S., a key market for hypothermia systems.
Similarly, in July 2023, ZOLL, an Asahi Kasei company, entered an exclusive distribution agreement with BrainCool for the BrainCool System/IQool System and IQool System Pads in the U.S. and select European and Asian markets. This deal aims to broaden access to advanced cooling technologies, particularly for neurological applications, and is expected to bolster ZOLL’s competitive position. Such partnerships are critical for manufacturers to navigate regulatory challenges and penetrate high-potential regions.
Regulatory and Reimbursement Dynamics
Regulatory support is a key driver of market growth. The U.S. Food and Drug Administration (FDA) and European regulatory bodies have streamlined approval processes for hypothermia systems, recognizing their clinical benefits. Recent FDA approvals for next-generation cooling devices have encouraged manufacturers to innovate, with companies like Stryker and Terumo Corporation launching updated systems in 2025. These devices comply with stringent international standards, facilitating market entry in regions with diverse regulatory frameworks.
Reimbursement policies are also evolving to support therapeutic hypothermia. In developed markets, favorable reimbursement for TTM procedures is encouraging hospitals to adopt these systems. However, challenges remain in developing regions, where limited healthcare budgets and inconsistent reimbursement policies can hinder adoption. Manufacturers are addressing this by offering cost-effective systems tailored to emerging markets.
Regional Market Trends
North America remains the largest market for therapeutic hypothermia systems, driven by advanced healthcare infrastructure and high incidence of cardiac arrest and stroke. The U.S., in particular, benefits from robust research and development (R&D) ecosystems and widespread adoption of TTM protocols. Europe follows closely, with countries like Germany and the UK investing in hypothermia systems for neonatal and neurological care.
The Asia-Pacific region is emerging as a high-growth market, fueled by increasing healthcare expenditure and rising awareness of hypothermia’s benefits. Countries like China and Japan are seeing growing demand for cooling systems in urban hospitals. However, market penetration in rural areas remains limited due to infrastructure constraints and high costs. Manufacturers are focusing on localized production and partnerships to address these challenges.
Challenges and Competitive Landscape
Despite its growth, the therapeutic hypothermia systems market faces challenges. High costs of advanced systems can deter adoption, particularly in resource-constrained settings. Additionally, the complexity of implementing TTM protocols requires specialized training, which can be a barrier for smaller healthcare facilities. Data privacy concerns, especially with AI-integrated systems, also pose regulatory and ethical challenges.
The competitive landscape is dynamic, with key players like Asahi Kasei Corporation, Gentherm Incorporated, Stryker Corporation, and Terumo Corporation leading the market. These companies are investing in R&D to develop next-generation systems with enhanced precision and portability. Smaller players, such as Shenzhen Comen Medical Instruments and Phoenix Medical Systems, are carving out niches by offering affordable solutions for emerging markets.
Impact of Global Health Trends
The rising prevalence of cardiovascular diseases and strokes is a significant driver of demand for therapeutic hypothermia systems. Aging populations in developed countries and increasing lifestyle-related health issues in emerging markets are contributing to this trend. Additionally, the integration of hypothermia systems with telehealth and remote monitoring technologies is aligning with broader healthcare trends, enabling real-time patient management and improving accessibility.
The market is also benefiting from increased investment in clinical research. Ongoing trials are exploring new applications for hypothermia, such as in traumatic brain injury and oncology, which could further expand the market’s scope. These studies are supported by government initiatives and orphan drug designations, which incentivize R&D in rare and critical conditions.
Conclusion
The therapeutic hypothermia systems market in 2025 is poised for growth, driven by clinical acceptance, technological advancements, and strategic partnerships. Innovations like AI integration and portable cooling systems are enhancing the efficacy and accessibility of these devices, while regulatory support and reimbursement policies facilitate adoption. However, challenges such as high costs and training requirements must be addressed to ensure equitable access, particularly in emerging markets. As healthcare systems prioritize patient outcomes in critical care, therapeutic hypothermia systems will remain a cornerstone of emergency medicine, with ongoing research and partnerships shaping a promising future.















Write a comment ...